pH sensor
| URL | https://www.bco-dmo.org/dataset/661659 |
|---|---|
| Download URL | https://www.bco-dmo.org/dataset/661659/data/download |
| Media Type | text/tab-separated-values |
| Created | October 14, 2016 |
| Modified | June 12, 2019 |
| State | Final no updates expected |
| Brief Description | Sulfate reduction energetics at Main Endeavor grotto chimney. |
Acquisition Description
Tables and Figures referenced in the acquisition description are found in the paper Frank et al., 2015
For each independent treatment, aliquots of 7.5 mL flange slurry (approx. 29 g wet weight and 20 g dry weight) were transferred into Balch tubes in an anaerobic chamber, and supplemented with 15 mL of sterile artificial seawater media designed to mimic the geochemical conditions within a hydrothermal flange (400 mM NaCl, 25 mM KCl, 30 mM CaCl2, 2.3 mM NaHCO3, 14 mM NaSO42-, 1 mM H2S, and 50 uM dissolved organic carbon – consisting of equimolar proportions 10 uM of pyruvate, citrate, formate, acetate, lactate) under a pure nitrogen headspace.
Concentrations of sulfide, sulfate and dissolved organic carbon (DOC) were varied independently to investigate concentration dependent effects on the rates of SR. The range of experimental conditions tested was determined from previously published concentration profiles of aqueous species modeled as functions of temperature and position within the Grotto vent structure (Tivey, 2004). Concentrations were varied by orders of magnitude within the modeled ranges to simulate conditions representative of different mixing regimes between seawater and vent fluid (Table 1). The range of DOC (which we approximate as a mix of pyruvate, citrate, formate, acetate, lactate – most of which have been identified to varying degrees within vent fluid and are known carbon sources for heterotrophic SR in culture) concentrations tested were based on the average DOC concentrations measured within diffuse fluids at the Main Endeavor Field (Lang et al., 2006; Lang et al., 2010). Hydrogen sulfide was present as H2S (pKa in seawater of 6.60) across all the conditions tested (Amend & Shock, 2001). Incubations were carried out at pH 4 (to simulate the pH of end-member Grotto vent fluid and the average calculated pH of mixed fluids in highly reduced zones within the flange; Tivey 2004) as well as pH 6 (representative of the calculated pH in fluid mixing zones; Tivey 2004). All the results are presented and discussed in the context of the initial measured media conditions.
Processing Description
Tables and Figures referenced in the processing description are found in the paper Frank et al., 2015
Potential energy yields of the different metabolisms available in the incubations depend on temperature and fluid compositions. To quantify the energy yield from heterotrophic sulfate reduction (Table 2) in each incubation values of overall Gibbs energy () were calculated according to:
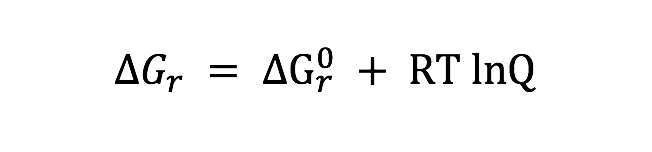
where is the standard Gibbs energy of reaction at in situ temperature and pressure conditions, R is the gas constant, T is the temperature (Kelvin), and Q is the activity product, defined as
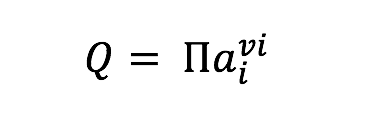
where ai represents the activity of the ith species and vi is the stoichiometric reaction coefficient, which is positive for products and negative for reactants. Values of were calculated at 1 bar and incubation temperatures using the geochemical software package SUPCRT92 (Johnson et al., 1992) and additional thermodynamic data from (Shock, 1995). Activities of aqueous species were calculated using the geochemical speciation program EQ3 (Wolery, 1992) based on the media composition described in section 2.2 and Table 1, with additional data from previously published work (Shock, 1995; Shock & Koretsky, 1993). For concentrations equal to zero, a value of 10-13 mol/kg was used as input. Resulting aqueous activities were used to calculate values of normalized for the number of electrons transferred in the redox for the reactions in Table 2. These reflect the metabolic energy available at the start of each incubation experiment for the complete oxidation of each organic acid, metabolisms that are documented among known sulfate reducers (Amend and Shock, 2001). Furthermore, to calculate the energy density in each incubation (as in Amend et al., 2011), it was assumed that the amended organic acids were the limiting reactant for all experiments when sulfate concentrations were in excess of 1 mM; otherwise sulfate was assumed to be limiting. While some sulfate reducers are known to produce carboxylic acid and alcohol intermediates, incomplete oxidation reactions were not considered here, as the goal of these calculations was to generate a broad understanding of sulfate reduction energetics, and not the metabolic potential for a particular species. Such an approach is common when comparing microbial metabolisms independent of species-specific pathways (e.g. Amend et al., 2004; Rogers & Amend, 2006; Skoog et al., 2007), although it should be noted that incomplete oxidation (fermentation) generally yields much less energy than complete oxidation (Rogers & Amend, 2006; Skoog et al., 2007).
To account for potential interactions between chimney-derived trace metals and amended sulfide, the saturation states of sulfide minerals were calculated as part of the initial fluid speciation. Using reported concentrations of relevant trace metals (Fe, Zn, Cu, etc.) in end-member Grotto hydrothermal fluid (Butterfield et al., 1994), maximum aqueous activities of trace metals were calculated with the EQ3 geochemical speciation program (EQ3/6 1998; EQ3NR 1998). Several sulfide minerals commonly found in hydrothermal chimneys (e.g. pyrite, chalcocite, sphalerite) were supersaturated under incubation conditions, particularly for incubations with high concentrations of amended sulfide. The irreversible abiotic precipitation of mineral sulfides has the potential to draw down aqueous sulfide concentrations and impact sulfate reductions rates. Therefore, the geochemical reaction path program EQ6 (EQ3/6 1998; EQ6 1998) was used to constrain fluid compositions to equilibrium with these minerals phases. Using the single point model in EQ6, the Gibbs energy of the system was allowed to reach local minima by mineral precipitation, however redox reactions among carbon and sulfur species was suppressed with a custom thermodynamic database. The resulting fluid compositions were used to calculate metabolic reaction energetics as well as to evaluate the potential effects of metal speciation on sulfate reduction rates.
BCO-DMO Data Processing Notes:
-reformatted column names to comply with BCO-DMO standards
-filled in all blank cells with nd
-removed spaces and replaced with underscores
Instruments
DOC was measured
Used aboard ship and in lab
A device on shipboard or in the laboratory that holds water samples under controlled conditions of temperature and possibly illumination.
Parameters
concentration of sulfide
Concentration of sulfate (SO4) per unit volume
Concentration of bicarbonate ion ([HCO3]-) in seawater. Refer to dataset for units of measure.
K (potassium) concentration. May be reported in parts per million, nanomoles/Liter, or other units. Refer to dataset metadata for units.
Calcium (Ca). Concentrations may be reported in parts per million, nanomoles per liter, or other units. Refer to dataset metadata for units.
Sodium (Na). Concentrations may be reported in parts per million, nanomoles per liter, or other units. Refer to dataset metadata for units.
pH: The measure of the acidity or basicity of an aqueous solution
Temperature in degrees C of a sample or other item. A generic temperature measurement.
Note: This is NOT water temp or sea surface temp
Carbon limiting total energy available in bottle for sulfate reduction
Latitude
latitude, in decimal degrees, North is positive, negative denotes South; Reported in some datasets as degrees, minutes
Dataset Maintainers
| Name | Affiliation | Contact |
|---|---|---|
| Peter R. Girguis | Harvard University | |
| Karyn L. Rogers | Harvard University | |
| Kiana L. Frank | Rensselaer Polytechnic Institute (RPI) | |
| Hannah Ake | Rensselaer Polytechnic Institute (RPI) | |
| Shannon Rauch | University of Hawaii at Manoa (SOEST) | ✓ |
| Hannah Ake | Woods Hole Oceanographic Institution (WHOI BCO-DMO) |
BCO-DMO Project Info
| Project Title | Characterizing the distribution and rates of microbial sulfate reduction at Middle Valley hydrothermal vents |
|---|---|
| Acronym | Middle Valley Vents |
| URL | https://www.bco-dmo.org/project/626603 |
| Created | November 17, 2015 |
| Modified | November 19, 2015 |
Project Description
This project characterizes rates of microbially mediated sulfate reduction from three distinct hydrothermal vents in the Middle Valley vent field along the Juan de Fuca Ridge, as well as assessments of bacterial and archaeal diversity, estimates of total biomass and the abundance of functional genes related to sulfate reduction, and in situ geochemistry. Maximum rates of sulfate reduction occurred at 90°C in all three deposits. Pyrosequencing and functional gene abundance data reveal differences in both biomass and community composition among sites, including differences in the abundance of known sulfate reducing bacteria. The abundance of sequences for Thermodesulfovibro-like organisms and higher sulfate reduction rates at elevated temperatures, suggests that Thermodesulfovibro-like organisms may play a role in sulfate reduction in warmer environments. The rates of sulfate reduction observed suggest that – within anaerobic niches of hydrothermal deposits – heterotrophic sulfate reduction may be quite common and might contribute substantially to secondary productivity, underscoring the potential role of this process in both sulfur and carbon cycling at vents.
This project was funded, in part, by a C-DEBI Graduate Student Fellowship.
Data Project Maintainers
| Name | Affiliation | Role |
|---|---|---|
| Peter R. Girguis | Harvard University | Principal Investigator |
| Kiana L. Frank | University of Hawaii at Manoa (SOEST) | Contact |

